Infliximab Biosimilar List
biosimilar infliximab list wallpaperThe others are stalled by legal delays. As of June 2020 14 biosimilars have been approved to treat inflammatory arthritis but only three are available for doctors to prescribe.
 Tumor Necrosis Factor Tnf Inhibitors A Clinical Primer
Tumor Necrosis Factor Tnf Inhibitors A Clinical Primer
The approval of biosimilar products can improve access to care for patients by increasing the number of medication options and potentially lower costs.

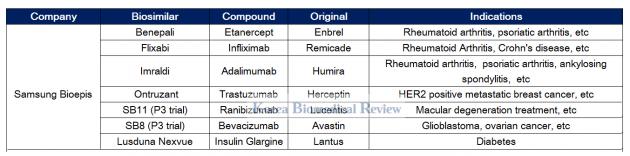
Infliximab biosimilar list. Major players in the adalimumab infliximab and etanercept biosimilars market are Zydus Cadila Sandoz Novartis Samsung Bioepis AbbVie Amgen Boehringer Ingelheim Pfizer Celltrion and Mylan. Remicade infliximab Avsola Information. Adult Crohn disease management after surgical resection.
Infliximab is a chimeric monoclonal antibody against tumour necrosis factor alpha TNF-α. Avsola infliximab-axxq Amgen a biosimilar to Remicade infliximab Janssen is a tumor necrosis factor blocker intended for patients with rheumatoid arthritis in combination with. We currently market Zarzio 2 Zarxio 2 Binocrit 3 and Ziextenzo 4 used in supportive cancer care and Rixathon 5 to treat blood cancers.
In 2017 the biosimilars Amgevita Solymbic Imraldi and Cyltezo were approved for use in the European Union. Infliximab-axxq Avsola TM infliximab-dyyb Inflectra and infliximab-abda Renflexis are biosimilar murine-human chimeric monoclonal antibodies to infliximab Remicade. It marks the third infliximab biosimilar to be approved in the US.
Food and Drug Administration today approved Inflectra infliximab-dyyb for multiple indications. Food and Drug Administration today approved Inflectra infliximab-dyyb for multiple indications. In the patent infringement action between Janssen and Hospira relating to the biosimilar INFLECTRA the Federal Court of Appeal released its decision on January 30 2020 remitting for reconsideration to the trial judge certain issues relating to the validity of Janssens patent see our article here.
Biosimilar treatment options could free up resources which can be redeployed for innovation access and new interventions within healthcare systems to support and manage the burden of oncology care. They are infliximab-axxq Avsola infliximab-dyyb Inflectra and infliximab-abda Renflexis and biosimilars to Remicade. Inflectra is administered by intravenous infusion.
Biosimilars are highly similar but nonidentical biologic agents with no differences in clinical efficacy and safety when compared to bio-originator pr. This includes Crohns disease ulcerative colitis rheumatoid arthritis ankylosing spondylitis psoriasis psoriatic arthritis and Behçets disease. In Canada Avsola Inflectra and Renflexis are also approved as biosimilars to Remicade infliximab.
This is the second biosimilar approved by the FDA. The originator product Johnson Johnson and Mercks Remicade infliximab was approved by the US Food and Drug Administration FDA in August 1998 and by the European Medicines Agency EMA in August 1999 1. Ixifi infliximabqbtx has been approved in all eligible indications of the originator product Remicade which includes rheumatoid arthritis Crohns disease paediatric Crohns disease ulcerative colitis ankylosing spondylitis psoriatic arthritis and plaque psoriasis.
Both Remicade and its biosimilars infliximab-axxq Avsola TM inflixmab-dyyb. Janssen has applied to the. Janssen has applied to the.
Inflectra is biosimilar to Janssen Biotech Incs Remicade infliximab which was originally licensed in 1998. This is the second biosimilar approved. Remicade infliximab Biosimilars Inflectra infliximab-dyyb Renflexis infliximab-abda Ixifi infliximab-qbtx Avsola infliximab-axxq.
TNF-α receptor activation is prevented by IFX through binding to TNF-α thereby neutralizing its biological activity. Infliximab a chimeric monoclonal antibody sold under the brand name Remicade among others is a medication used to treat a number of autoimmune diseases. In 2018 the biosimilars Halimatoz Hefiya Hyrimoz and Hulio were approved for use in the European Union.
Inflectra is administered by intravenous infusion. Infliximab IFX is a chimeric humanmurine monoclonal antibody that binds with high affinity to both soluble and transmembrane forms of tumour necrosis factor alpha TNF-α. In the patent infringement action between Janssen and Hospira relating to the biosimilar INFLECTRA the Federal Court of Appeal released its decision on January 30 2020 remitting for reconsideration to the trial judge certain issues relating to the validity of Janssens patent see our article here.
It is used to treat autoimmune diseases such as ankylosing spondylitis Crohns disease psoriasis psoriatic arthritis rheumatoid arthritis and ulcerative colitis.
Firstword Lists Which Biologics Face The Most Competition From Biosimilars Firstword Biosimilars
Https Www Cadth Ca Sites Default Files Symp 2017 Presentations April24 2017 Concurrent Session B4 Gary Warwick Pdf
 Who Wants The Biggest Slice Of The Biosimilar Pie The Humira Biosimilar Wave In Europe Eversana
Who Wants The Biggest Slice Of The Biosimilar Pie The Humira Biosimilar Wave In Europe Eversana

 Fda Approves Renflexis As Second Infliximab Biosimilar Rheumnow
Fda Approves Renflexis As Second Infliximab Biosimilar Rheumnow
 How The U S Compares To Europe On Biosimilar Approvals And Products In The Pipeline Biosimilars Law Bulletin
How The U S Compares To Europe On Biosimilar Approvals And Products In The Pipeline Biosimilars Law Bulletin
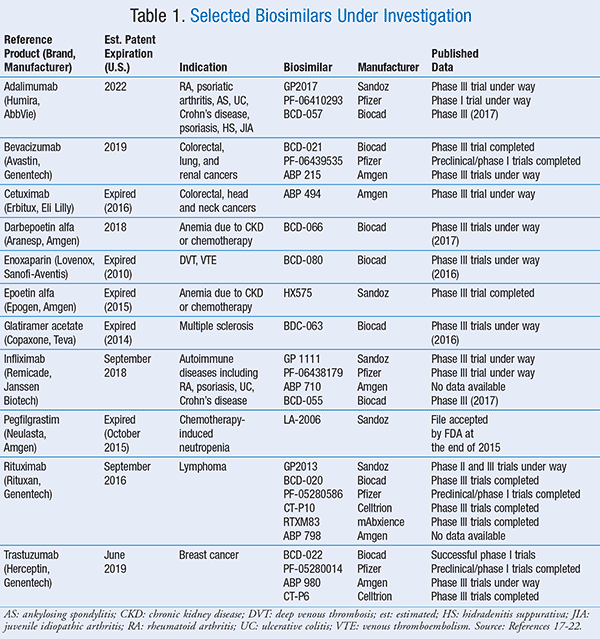 Biosimilars Current Approvals And Pipeline Agents
Biosimilars Current Approvals And Pipeline Agents
 The U S Biosimilars Market Shaking The Laggard Label Contract Pharma
The U S Biosimilars Market Shaking The Laggard Label Contract Pharma
 The Top 5 Biosimilars Articles For The Week Of July 13 Center For Biosimilars
The Top 5 Biosimilars Articles For The Week Of July 13 Center For Biosimilars

 Poster 6 2017 Potential Savings From Biosimilars In Canada
Poster 6 2017 Potential Savings From Biosimilars In Canada
 Samsung Bioepis And Merck Launch Renflexis In The U S Biosimilars Law Bulletin
Samsung Bioepis And Merck Launch Renflexis In The U S Biosimilars Law Bulletin
 Current Anti Tnf Biologics Including Biosimilars And Their Biological Download Scientific Diagram
Current Anti Tnf Biologics Including Biosimilars And Their Biological Download Scientific Diagram
Cube Rm Insights Trends Regarding Tendering Of Biosimilars In Europe Cube Rm
 How The U S Compares To Europe On Biosimilar Approvals And Products In The Pipeline Biosimilars Law Bulletin
How The U S Compares To Europe On Biosimilar Approvals And Products In The Pipeline Biosimilars Law Bulletin
 How The U S Compares To Europe On Biosimilar Approvals And Products In The Pipeline Biosimilars Law Bulletin
How The U S Compares To Europe On Biosimilar Approvals And Products In The Pipeline Biosimilars Law Bulletin
Remicade Infliximab Biosimilar Clinical Trial Insight
 Biosimilars Law Bulletin Rothwell Figg Biologics And Biosimilars Practice
Biosimilars Law Bulletin Rothwell Figg Biologics And Biosimilars Practice
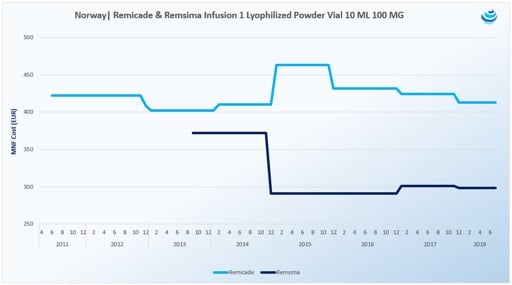
 Biosimilar Pricing In Europe Eversana
Biosimilar Pricing In Europe Eversana